SOIL pH
February 04, 2025
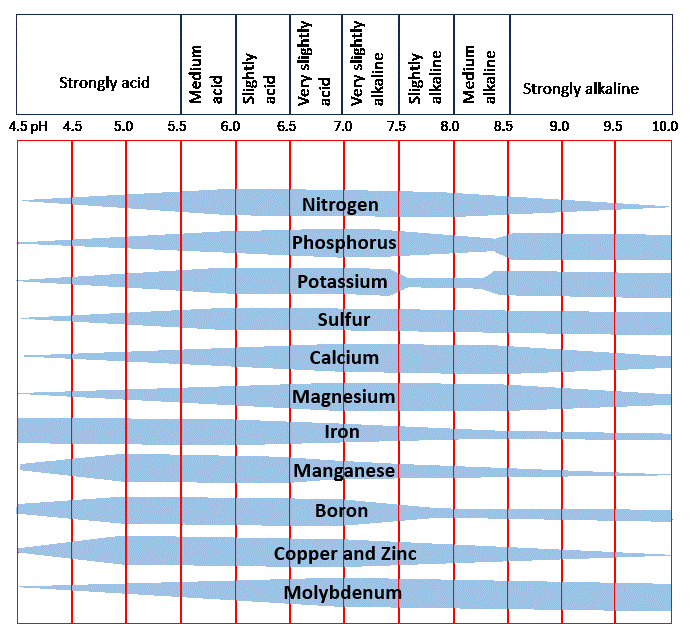
Soil contains air, water, minerals, and microorganisms. It serves as a nutrient store while holding plant roots in place. Soil pH is an important indicator, that tells us about the suitability of a soil for plant growth. When Soil pH levels are too high or too low the soil cannot continue to serve the needs of the plants. In other words, nutrients taken up by plant roots are not good enough for the survival of the plants.
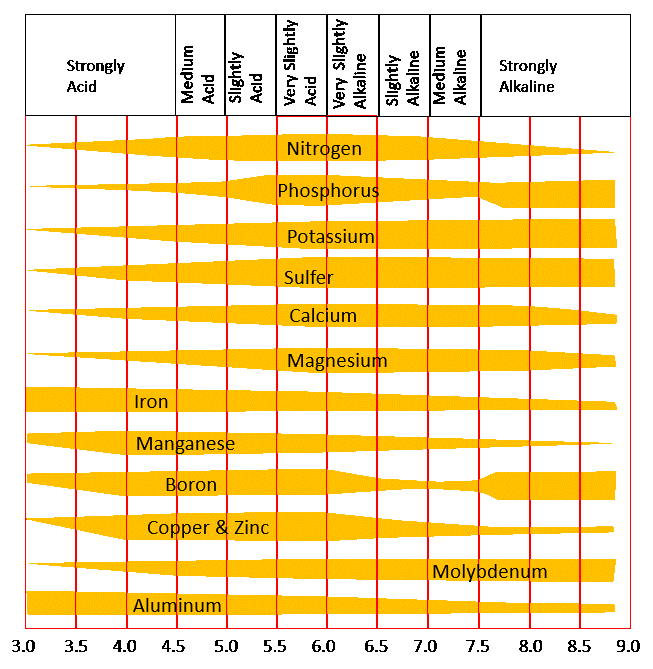
A soil with a pH below 7 is acidic. Compared to pH 7, pH 6 is 10 times more acidic, and pH 5 is 100 times more acidic. Soil pH levels influence the solubility of the nutrients—iron, magnesium, phosphorus, calcium. At low pH levels, not as many of the beneficial elements are available for the roots to take up. However, at low pH levels availability of other elements; like aluminum, increases and may become toxic to plants.
Soil pH levels that are too high lead to a decrease in crop yields. High pH levels can limit the availability of manganese, copper, zinc, phosphorus, and boron, as well.
Legume root colonizing bacteria (Rhizobia) have preferred soil pH ranges in which they are effective. When the soil pH level is too low, organisms which contribute to the availability of nitrogen disappear. Also, the microorganisms need nitrogen in the soil to compost organic matter. When microbial activity declines, organic matter is not well-composted.
Land use—conversion of land from forestland or grassland to cropland, affects the soil pH and, may result in drastic changes in pH over time. High amounts of rainfall, in warm, humid environments, can cause soil pH to decrease over time. However, soils with a high content of clay and organic matter have a higher buffering capacity—and are more resistant to changes in pH.
pH measurements help improve soil health. You can measure Soil pH levels yourself using a cheap and easy-to-use DIY kit. Alternatively, for detailed analysis, send a soil sample to a laboratory. Laboratories use two methods to measure pH levels. The result of the distilled water method is expressed as pH (w). The calcium chloride test—the result is typically expressed as pH (CaCl2), which is considered more accurate. pH (CaCl2) is lower than pH (w) by about 0.5 – 0.9.
It is important to note that the optimum pH is not the same for all crops. However, most crops grow best in the pH range of 6 to 7.5. A soil pH (CaCl2) ranges from 5.2 to 7.5 and provides optimum conditions for many plants.
More →
- * pH (NRCS - United State Department of Agriculture)
- ** Understanding Soil pH (Belinda Lake, Yanco Agricultural Institute)
- SOIL pH (Spokane County Extension - Washington State University)
